Cancers We Treat

Learn More

We use state-of-the-art surgical technology for safer procedures and faster recovery times.

Take our online quiz and get a free, personalized report that helps you understand your risk.
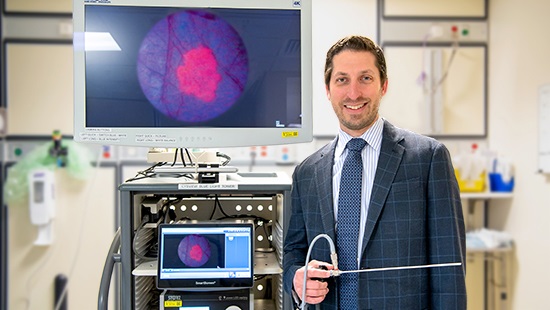
An ultraviolet light that causes tumors to fluoresce is used during cystoscopy, allowing for more accurate removal.
The Tate Cancer Center uses the latest, minimally invasive technology for safer treatment and faster recovery, and our cancer care specialists work together to ensure a comprehensive treatment plan for each patient.
Our Cancer Center is also affiliated with the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center (UMGCCC), and is part of the University of Maryland Cancer Network. The Network provides access to national experts, the latest treatments, leading edge technology, and promising clinical trials