University of Maryland Center for Aortic Disease First in Region to Use the TREO Endograft in Repairing an AAA
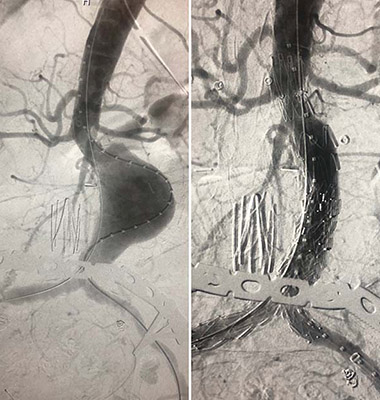
(A) An abdominal aortic aneurysm (AAA) before repair. (B) The same AAA after deployment of the TREO endograft.
Recently, a team at the University of Maryland (UM) Center for Aortic Disease was among the first three in the nation - all on the same day - and the first in the mid-Atlantic region to complete an endovascular aortic abdominal aneurysm (AAA) repair with the TREO® endograft three-piece modular system (Terumo Aortic (US), formerly Bolton Medical, Sunrise, FL), which gained FDA approval this past May. The endovascular aortic aneurysm repair (EVAR) procedure was led by vascular surgeon Khanjan H. Nagarsheth, MD, Assistant Professor of Surgery at the University of Maryland School of Medicine.
“We are pleased to have had the TREO triple stent-graft available to us for this particular case,” says Dr. Nagarsheth. “It involved an aortic anatomy untreatable by other currently FDA-approved endografts, and this leading-edge device – which is designed to accommodate hundreds of configurations – allowed us to offer the patient an endovascular procedure.”
Depending on an individual patient’s needs, the UM aortic surgery team of cardiothoracic and vascular surgeons often use custom-made fenestrated endografts for aortic repair. Through advances in CT angiography, a detailed rendering of a patient’s diseased aorta and affected branching arteries can be 3D printed and sent to Cook Medical in Australia to construct the customized graft. However, this process takes several weeks and sometimes months. Therefore, when patients present with an aortic aneurysm that must be addressed urgently, surgeons must rely on commercial grafts, even if they do not fully conform to the degree of precision the case requires, or resort to open surgery, which may not be ideal for the patient.
Unlike other off-the-shelf endovascular devices, the three-piece TREO permits numerous configurations that can be customized to the patient’s anatomy more precisely. The TREO’s main stent-graft consists of a proximal section – the length of which can be adjusted when deployed into the aneurysmal sac – with a bifurcation to accommodate the iliac arteries. This main stent-graft is available in eight diameters ranging from 20 to 36 mm to better match the patient’s aortic anatomy. Through the bifurcated portion of the graft, two separate leg extensions are fitted and deployed into the iliac arteries. These extensions offer the surgeon distal diameter options of 9 mm, 11 mm and 13 mm so the appropriate size can be selected at each portion of the stented artery.
“The wide range of main graft diameters this system provides for will allow us to offer endovascular treatment to smaller patients with narrower aortic diameters,” says Dr. Nagarsheth, noting that many of these patients would require open aortic procedures were the TREO not available at the University of Maryland Center for Aortic Disease.
Dr. Nagarsheth adds that besides permitting a high-level of endograft customization, the TREO is designed with features that prevent the device’s migration. The proximal portion of the stent contains fixation barbs, with a second row of fixation barbs located farther down the main stent-graft. Additionally, the legs of the main stent-graft contain dull barbs that fixate them within the iliac arteries and keep the leg extension grafts locked into the main graft. This design prevents type 3 endoleaks and lends to the durability of the graft.
Studies into the TREO endograft performance look promising for continued good outcomes with the device. An Italian study of 37 patients found that, at a mean follow up of eight months – the device has not been available long enough to fully determine long term results – the TREO was associated with 100 percent technical success and a low reintervention rate (5.4%).1 Another measure of the overall success of EVAR is the resulting shrinkage of the aneurysmal sac, and there are reasons to believe the TREO facilitates this better than other commercial devices currently available. A retrospective study between TREO (n=6) and non-TREO (n=16) grafts performed at the University of Rochester in New York found that the shrinkage rate as well as the average size of shrinkage after EVAR was significantly greater in the group receiving a TREO graft.2
1Marone EM, Rinaldi LF, Lovotti M, Palmieri P, Argenteri A. The Bolton Treo endograft: a single-center preliminary experience. Ann Vas Surg. 2019 Apr;56:139-146. doi: 10.1016/j.avsg.2018.08.080.
2Balceniuk MD, Zhao P, Terbush MJ, Schroeder AC, et al. TREO aortic endograft demonstrates significant aneurysmal sac shrinkage. J Surg Res. 2019 Sep;241:48-52. doi: 10.1016/j.jss.2019.03.048
