UM Center for Aortic Disease at the Forefront of Innovation in the OR with Trial Research
Led by co-directors, Brad Taylor, MD, MPH, Professor of Surgery and Vice Chief, Division of Cardiac Surgery and Shahab Toursavadkohi, MD, Assistant Professor of Surgery, the University of Maryland Center for Aortic Disease is among the nation's top tier of centers for the treatment of aortic dissection and aneurysm. The Center's comprehensive array of aortic clinical trials helps ensure University of Maryland Medical Center (UMMC) surgeons provide definitive treatment for every patient presented to them with aortic disease including the more complex in nature. Only an elite group of the nation's hospitals currently offer access to as many state-of-the-art, investigational aortic repair devices as available at UMMC through participation in a clinical trial. For many patients, these early feasibility trials and pivotal trials are the reason they are alive today.
The following current prominent University of Maryland (UM) Center for Aortic Disease trials investigate leading-edge devices to supply a full gamut of minimally invasive, endovascular solutions for disease along any portion of the aorta, including the ascending aorta and the aortic arch. As such, these trials well-equip UM surgeons to provide definitive care, and ultimately hope, for all patients regardless of their case complexity or ability to tolerate surgery.
Ascending Aorta Trial
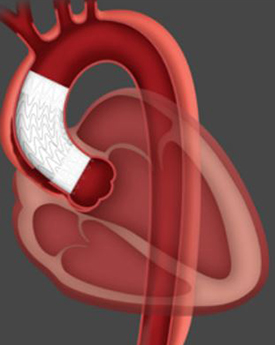
Trial Name: A Multicenter, Early Feasibility Study of the GORE® Ascending Stent Graft in Subjects with Type A Dissections of the Ascending Aorta [ARISE]
NCT Number: NCT02380716
Description: Without treatment, half of all type A dissections (located in the ascending aorta) are fatal within 48 hours,1 and they are particularly challenging to definitively treat because 1 in about every 5 patients with a type A dissection is too ill to tolerate a surgical procedure.2 With medical management alone, about 60 percent of patients with a type A dissection will die while hospitalized, and 90 percent will die within a year.3 While endovascular repair is now the standard for most type B dissections (descending aorta), this approach is still in pioneer stages for the ascending aorta, not the least because of a paucity of repair devices to treat the structure of the ascending aorta.
The ARISE trial seeks to change that by exploring endovascular treatment options for pathologies of the ascending aorta. Conducted in the United States under an Investigational Device Exemption (IDE), the trial initially utilized the GORE® TAG® thoracic branch endoprosthesis as a solution for type A dissection and is now investigating the GORE Ascending Stent Graft. Specifically tailored for the ascending aorta, the device is the first to consider the structure and curvature of this aortic segment. As with other GORE® aortic devices, the delivery system allows doctors to angulate the device to ensure optimal placement and patency.
Aortic Arch/Descending Aorta Trials

Trial Name: A Prospective, Multicenter, Non-Blinded, Non-Randomized Early Feasibility Study of the RelayBranch Thoracic Stent-Graft System in Subjects with Thoracic Aortic Pathologies Requiring Treatment Proximal to the Origin of the Innominate Artery
NCT Number: NCT03214601
Description: The first trial of a double branch system for treating pathologies of the aortic arch and proximal descending aorta in the US, this early feasibility study of Terumo Aortic's (formerly Bolton Medical's) RelayBranch offers patients an endovascular treatment for aneurysmal disease, penetrating atherosclerotic ulcer (PAU) or chronic uncomplicated Type B aortic dissection.
The RelayBranch system comprises a main body graft similar in design to that of the RelayPro (see "Descending Aorta Trials" below). The surgeon deploys this main graft in the descending aorta before deploying two branch grafts into the brachiocephalic artery and left common carotid artery. As a follow-up to the index procedure, a small open procedure is performed to reperfuse the left subclavian artery, completing the treatment.
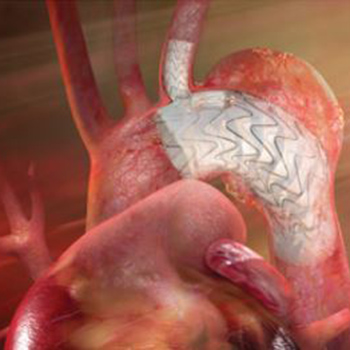
Trial Name: Evaluation of the GORE® TAG® Thoracic Branch Endoprosthesis (TBE Device) in the Treatment of Lesions of the Aortic Arch and Descending Thoracic Aorta
(Zone 0/1)
NCT Number: NCT02777528
Description: This safety and feasibility trial studies the GORE®TAG®Thoracic Branch Endoprosthesis (TBE) device, which consists of a descending thoracic aortic graft and a single branch graft used to treat the branch artery adjacent to the diseased aortic arch. In this trial, the TBE device will be used to repair zone 0/1 aortic aneurysms (arm 1) and non-aneurysm lesions, including dissections and intramural hematomas (arm 2). After TBE device placement, patients will undergo a revascularization procedure to reconnect any aortic branch arteries occluded by the graft.
DESCENDING AORTA TRIALS RelayPro-D Thoracic Stent-Graft Trials

Trial Name: A Prospective, Multicenter, Non-Blinded, Non-Randomized Study of the RelayPro Thoracic Stent-Graft in Subjects with an Acute, Complicated Type-B Aortic Dissection
NCT Number: NCT03033043
Trial Name: A Prospective, Multicenter, Non-Blinded, Non-Randomized Study of the RelayPro Thoracic Stent-Graft in Subjects with Traumatic Injury of the Descending Thoracic Aorta
NCT Number: NCT03090230
Description: Two aortic trials at the UM Center for Aortic Disease assess the safety and efficacy of Terumo Aortic's RelayPro Thoracic Stent-Graft device: the first in subjects with acute, complicated type-B dissections and the second in subjects with traumatic injuries in the descending abdominal aorta. The device delivery system's straw-width profile allows for stent placement in the descending aorta even in cases with access challenges, and its flexible, nitinol inner catheter reduces access-related damage and injury. Moreover, the RelayPro stent-graft's varying lengths, widths, and bare and non-bare proximal configurations provide surgeons with multiple structural options to achieve patency.
While many endovascular aortic repair device feasibility trials follow patients for only 30 days after the index procedure, these two RelayPro trials continue to measure treatment success at six months, 12 months and annually thereafter for up to five years.
Endovascular Aortic Repair Study
Trial Name: Global Registry for Endovascular Aortic Treatment
NCT Number: NCT01658787
Description: To track the performance and clinical outcomes of all Gore® Endovascular Aortic devices, including those used for abdominal as well as thoracic aortic repair, a prospective, observational registry has been set up. Initiated in December 2010, to date this study has already enrolled upwards of 4,600 participants with a variety of aortic pathologies, and is estimated to be completed in 2026. Patients in the registry are followed for 10 years with the goal of collecting "'real world' data on the incidence of serious device events."
1Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011 Dec;58(24):2455-74. doi. 10.1015/j.jacc.2011.06.067.
2 Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015 Jul;66(4):350-8. doi: 10.1016/j.jacc.2015.05.029.
3 Ibid.
For more details about any of these trials or to refer a patient for aortic repair, call the UM Center for Aortic Disease at 410-328-4771 or view information for referring physicians.
Related Content
- Learn more about the UM Center for Aortic Disease
- View more recent articles from Dr. Taylor
- View more recent articles from Dr. Toursavadkohi
- Schedule Grand Rounds for your hospital with Dr. Taylor or Dr. Toursavadkohi
- Meet our multidisciplinary UM Center for Aortic Disease Team
