Researchers Set Sights on Targeting CTC Microtentacles to Reduce Cancer Metastasis
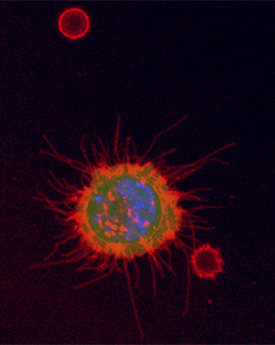
Next to two smaller red blood cells, a tumor cell with microtentacles is chemically fixed by the TetherChip.
That circulating breast tumor cells (CTCs) form and use tubulin-based, dynamic, protrusive microtentacles to invade and metastasize to other tissues was first described in 2008 by a team led by Stuart S. Martin, PhD, the Drs. Angela and Harry Brodie Professor in Translational Cancer Research at the University of Maryland School of Medicine and co-leader of the Hormone Responsive Cancer Program at the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center (UMGCCC).1
Because different oncogenes drive tumor growth and the proliferation of CTCs, some tubulin-stabilizing agents (e.g., Paclitaxel) used to shrink primary tumors can cause shedding of viable CTCs into the bloodstream, inadvertently contributing to metastasis.2
“Is the tumor getting smaller because cells are dying,” asks Martin, “or because cells are breaking free and spreading? These are questions we are seeking to answer as we gain more knowledge about epithelial circulating tumor cells and cancer metastasis.”
Primary tumors begin shedding CTCs very early in the disease process, and Martin says that more cancer research needs to focus on ways to prevent metastasis, of which targeting microtentacles is simply one strategy. After all, most patients who die of breast cancer perish from secondary metastatic disease rather than the primary tumor.
“There are very few therapies for stopping tumor spread in large part because clinical imaging isn’t able to see circulating tumor cells,” says Martin, who mentions that historically cancer research has focused on the size of primary tumors. “By the time a tumor appears on an MRI, it consists of 100 million cancer cells or more,” he continues, adding that even small tumors can shed a million CTCs into the bloodstream per day. Additionally, as their detection methods grow more sensitive, researchers in Martin’s lab and others are learning that any modality of initial treatment of the primary tumor has the potential to exacerbate CTC shedding and, moreover, may be making the remaining primary tumor cells even more dangerous.
Thankfully, the inhospitable environment of the bloodstream, complete with capillaries narrower than the tumor cells that can potentially break them up, means that a very large majority of CTCs are killed before they have an opportunity to embed in healthy tissue.
“Metastasis is an inefficient process,” says Martin. However, he adds that when clusters of CTCs appear, they have a metastatic potential up to 50 times greater than that of single circulating tumor cells, perhaps because the outer cells of the cluster protect the inner cells. “Microtentacles help CTC cluster together, so if we can use either approved drugs or develop new ones to inhibit the microtentacles on CTCs, we can prevent these clusters from forming,” Martin continues.
Prevalence of Microtentacles & How They Assist Metastasis
Since Martin’s discovery that breast cancer CTCs possess microtentacles, his lab has also detected them in the CTCs of colon, lung and prostate cancers. Another UMGCCC group of researchers has confirmed that circulating ovarian tumor cells also develop microtentacles, and a group at the University of California Berkeley has found them on glioblastoma brain cancer cells. It is feasible that any cancer that develops from epithelial cells – representing the 90 percent of solid malignant tumors called carcinomas – could possess microtentacles given that they appear to arise from the inherent wound-healing process in epithelial cells.
Once tumor cells break free from the primary tumor, microtentacles assist the CTCs in attaching to vessel walls, where they then have the potential to escape from the circulatory or lymphatic systems and into hospitable tissue. While they are incompatible with most differentiated tissues, it is common for CTCs, sometimes circulating in the bloodstream for years, to eventually return to the tissue from which they originated. Such is the case in “self-seeding” cancers and is why even patients who had oncologic surgeries in which zero margins are achieved can experience recurrence in the original tissue.
Overcoming the Challenges of Studying Microtentacles with the TetherChip
Microtentacles’ role in metastasis and even their existence went undetected until relatively recently because they are extremely thin – about 100 nm in width. Therefore, when a chemical fixative is added to a specimen slide, microtentacles collapse, effectively destroyed and rendered unseen on a microscope.
To overcome the practical challenges of studying the function and identify potential vulnerabilities of microtentacles, Martin collaborated with Christopher M. Jewell, PhD, the Minta Martin Professor of Engineering at the University of Maryland A. James Clark School of Engineering, to develop the TetherChip, a multilayer slide that effectively tethers free-floating cells so intact microtentacles can be observed through microscopy. The slide combines a multilayer nanosurface of clear, thermal-crosslinked polyelectrolytes with a thin hydrophobic lipid coating that tethers cell membranes while allowing cells to maintain their free-floating shape. Moreover, the TetherChip allows microtentacled cells to be chemically-stabilized for later study. The paper describing the TetherChip indicates that the device has a shelf-life of at least six months,3 and Martin says the group has now demonstrated that the TetherChip device remains stable for more than a year.
“We can now create fixed, archival samples of tumor cells with microtentacles and send them to multiple labs,” he says. “We need more researchers to work on the frontiers that we have only begun to clear.” Martin’s lab focuses primarily on hormone-responsive cancers such as breast and prostate tumor cells, although they are also collaborating with the University of California Berkeley group in studying glioblastoma.
Microtentacles as Indicators of Patient Prognosis
The presence of many CTCs with microtentacles as independent prognosticators of metastatic risk has been confirmed by mouse models and is under intense investigation for human patients. Martin’s lab has found that patients who had primary breast tumors with cells exhibiting high levels of -tubulin acetylation tended to have aggressive, metastatic disease and lower survival rates. Acetylated a-tubulin had an effect on microtentacle frequency as well as CTCs’ ability to reattach. The researchers believe that high-intensity acetylated -tubulin may even be a prognostic biomarker for breast cancer.4
Moving Towards Potential Strategies to Prevent Successful Metastasis
Microtentacles are supported by the cell’s microtubules and, in fact, are believed to be created when the outward force of microtubules exceeds the inward force of the cell membrane.5 Early on, Martin’s lab revealed that the microtubule-associated protein tau promotes microtentacles.6 Therefore agents that break down microtubules also cause instability for microtentacles; conversely, agents that stabilize microtubules – even if these same agents are efficacious in reducing primary tumor size – are potentially dangerous for cancer spread because they strengthen microtentacles.
In in a forthcoming proof-of-concept paper, Martin’s group will reveal an FDA-approved agent that targets CTC microtubules and successfully enhances survival rates in murine models. It is hoped the agent could one day be an adjunctive to first line cancer treatment regimens to reduce the chances of metastasis and recurrence.
For inquiries about the Hormone Related Cancer Program at UMGCCC, contact Stuart S. Martin.
Related Content
- UM School of Medicine Researchers Develop Novel Test for “Microtentacles” on Breast Cancer Cells
- UMMC Newsmakers – Microtentacles: How Breast Cancer Spreads
- Press Release: Stuart S. Martin Awarded New Endowed Professorship Honoring Internationally Renowned Breast Cancer Researcher Angela H. Brodie, PhD
- Hormone Related Cancers Program at the UM Greenebaum Comprehensive Cancer Center
1Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS. Vimentin filaments support extension of tubulin-based microtentacles in detached breast cancer cells. Cancer Res. 2008;68(14):5678-88. doi: 10.1158/0008-5472.CAN-07-6589
2Chakrabarti KR, Hessler L, Bhandary L, Martin SS. Molecular pathways: new signaling considerations when targeting cytoskeletal balance to reduce tumor growth. Clin Cancer Res. 2015;21(23):5209-5214. doi: 10.1158/1078-0432.CCR-15-0328
3Ju JA, Lee CJ, Thompson KN, Ory EC, et al. Partial thermal imidization of polyelectrolyte multilayer cell tethering surfaces (TetherChip) enables efficient cell capture and microtentacle fixation for circulating tumor cell analysis. Lab Chip. 2020;20:2872-2888. doi: 10.1039/D0LC00207K
4Boggs AE, Vitolo MI, Whipple RA, Charpentier MS, et al. -tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015;75(1):203-15. Doi: 10.1158/0008-5472.CAN-13-3563
5Matrone MA, Whipple RA, Balzer EM, Martin SS. Microtentacles tip the balance of cytoskeletal forces in circulating tumor cells. Cancer Res. 2010;70:7737-41. doi: 10.1158/0008-5472.CAN-10-1569
6Matrone MA, Whipple RA, Thompson K, Cho EH, et al. Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene. 2010;29(22):3217-27. doi: 10.1038/onc.2010.68
