Neurosurgery Clinical Research
MRI-Guided Focused Ultrasound Center
The University of Maryland Medical Center is among the first institutions to offer MRI-guided focused ultrasound (MRgFUS) to patients, with one of the highest volume practices in the country. Patients are treated weekly with thalamotomy for essential tremor. The center supports numerous large-scale trials that relate to movement disorders, neuropathic pain, and brain neoplasm, as well as various projects by neurosurgery residents. Drs. Eisenberg, Woodworth, and Gandhi serve as principle investigators in many of these projects.
Current Clinical Trials
- PD014 – Evaluation of the Safety and Effectiveness of Bilateral Exablate Ablation of the Pallidothalamic Tract (PTT) for the Treatment of the Motor Complications of Parkinson’s Disease (PD) (NCT04728295)
- TN – MR Guided Focused Ultrasound (FUS) for the Treatment of Trigeminal Neuralgia (NCT04579692)
- BT015 – Blood-Brain Barrier Disruption (BBBD) for Liquid Biopsy in Subjects With Glioblastoma Brain Tumors (NCT05383872)
- BT012 – Blood-Brain Barrier Disruption (BBBD) Using Exablate Focused Ultrasound With Standard of Care Treatment of NSCLC Brain Mets (NCT05317858)
Key Work
- Elias, W. J., et al. (2016). A randomized trial of focused ultrasound thalamotomy for essential tremor. New England Journal of Medicine, 375(8), 730-739.
Publications
Patient Experiences
Cerebrovascular and Endovascular Clinical Research
The department conducts a variety of cerebrovascular clinical research. In addition to contributing to several clinical trials, the department continues to investigate the use of low-dose heparin for the prevention of vasospasm after subarachnoid hemorrhage. There are strong collaborative ties and multiple ongoing clinical research projects with Neurointerventional Radiology, Neurology, and Radiation Oncology.
Current Departmental Clinical Trials
- MEMBRANE – Middle Meningeal Artery Embolization for the Treatment of Subdural Hematomas With TRUFILL® n-BCA (NCT04816591)
Key Work
- Simard, J. M., Aldrich, E. F., Schreibman, D., James, R. F., Polifka, A., & Beaty, N. (2013). Low-dose intravenous heparin infusion in patients with aneurysmal subarachnoid hemorrhage: a preliminary assessment. Journal of Neurosurgery, 119(6), 1611-1619.
- Hanley, D. F., et al. (2017). Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomized, multi-centre, multi-region, placebo-controlled CLEAR III trial. The Lancet, 389(10069), 603-611.
- Hanley, D. F., et al. (2019). Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. The Lancet, 393(10175), 1021-1032.
- Jindal, G., et al. (2019). Beyond the first pass: revascularization remains critical in stroke thrombectomy. Journal of Neurointerventional Surgery, 11(11), 1095-1099.
- Kole, M. J., et al. (2019). Pipeline embolization device diameter is an important factor determining the efficacy of flow diversion treatment of small intracranial saccular aneurysms. Journal of Neurointerventional Surgery, 11(10), 1004-1008.
Publications
- Marc Simard, MD, PhD
- Jacob Cherian, MD
- Dheeraj Gandhi, MD
- Timothy Miller, MD
- Gaurav Jindal, MD
- Mohamed Labib, MD
Neurotrauma Clinical Research
The department has a rich history of advancing the forefront of neurotrauma care, including contributing to the evaluation of decompressive craniotomy for traumatic brain injury, investigating the role of MRI in evaluating and treating spinal cord injury, and the utility of steroids for the treatment of brain and spine trauma. The department is a member of the North American Spinal Cord Injury Clinical Trials Network (NACTN) for Treatment of Spinal Cord Injury.
Current Departmental Clinical Trials
- Future clinical trials coming soon
Key Work
- Aarabi, B. and Simard, J.M., editors. Decompressive Craniectomy. Hauppauge, NY: Nova Science Publishers, 2018.
- Aarabi, B., et al. (2019). Extent of spinal cord decompression in motor complete (American Spinal Injury Association impairment scale grades A and B) traumatic spinal cord injury patients: post-operative magnetic resonance imaging analysis of standard operative approaches. Journal of Neurotrauma, 36(6): 862-876.
- Aarabi, B., et al. (2020). Efficacy of ultra-early (< 12 h), early (12-24 h), and late (> 24-138.5 h) surgery with magnetic resonance imaging-confirmed decompression in American Spinal Injury Association Impairment Grades A, B, and C cervical spinal cord injury. Journal of Neurotrauma, 37(3): 448-457.
- Bracken, M. B., et al. (1990). A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the Second National Acute Spinal Cord Injury Study. New England Journal of Medicine, 322(20), 1405-1411.
- Bracken, M. B., et al. (1990). A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the Second National Acute Spinal Cord Injury Study. New England Journal of Medicine, 322(20), 1405-1411.
- Cooper, D. J.,et al. (2011). Decompressive craniectomy in diffuse traumatic brain injury. New England Journal of Medicine, 364(16), 1493-1502.
- Eisenberg, H.M., et al. (2020). Magnetic resonance imaging pilot study of intravenous glyburide in traumatic brain injury. Journal of Neurotrauma. 37(1): 185-193.
- Eisenberg, H.M., et al. (1990). Initial CT findings in 753 patients with severe head injury: a report from the NIH Traumatic Coma Data Bank. Journal of Neurosurgery, 73(5): 688-698.
- Le, E., et al. (2015). Predictors of intramedullary lesion expansion rate on MR images of patients with subaxial spinal cord injury. Journal of Neurosurgery: Spine, 22(6), 611-621.
- Mushlin, H., et al. (2019). AOSpine subaxial cervical spinal injury classification system: the relationship between injury morphology, admission injury severity, and long-term neurologic outcome. World Neurosurgery, 130: e368-e374.
- Vaccaro, A. R., et al. (2016). AOSpine subaxial cervical spine injury classification system. European Spine Journal, 25(7), 2173-2184.
Publications
Spine Surgery Clinical Research
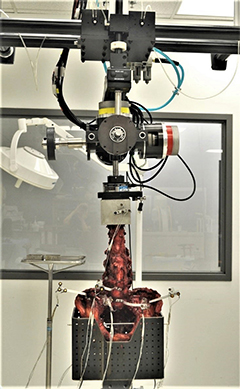
Drs. Sansur and Crandall support and mentor residents in numerous clinical research projects related to spine surgery, including degenerative disease and deformity, neoplastic disease, operative technique, and cadaveric studies of biomechanical fixation properties.
Key Work
- Sansur, C.A., et al. (2010). Morbidity and mortality in the surgical treatment of 10,242 adults with spondylolisthesis. Journal of Neurosurgery: Spine, 13(5): 589-593.
- Sansur, C.A., et al. (2011). Scoliosis research society morbidity and mortality of adult scoliosis surgery. Spine (Phila PA 1976), 36(9): E593-7.
- Maulucci, C.M., et al. (2016). Cortical bone facet spacers for cervical spine decompression: effects on intervertebral kinetics and foraminal area. Journal of Neurosurgery: Spine, 24(1): 69-74.
- Sansur, C. A., et al. (2016). Biomechanical fixation properties of cortical versus transpedicular screws in the osteoporotic lumbar spine: an in vitro human cadaveric model. Journal of Neurosurgery: Spine, 25(4), 467-476.
- Mushlin, H., et al. (2019). A biomechanical investigation of the sacroiliac joint in the setting of lumbosacral fusion: impact of pelvic fixation versus sacroiliac joint fixation. Journal of Neurosurgery: Spine, 31(4), 562-567.
- Wessell, A., et al. (2019). Thoracic discectomy through a unilateral transpedicular or costotransversectomy approach with intraoperative ultrasound guidance. Operative Neurosurgery (Hagerstown), 17(3): 332-337.
- Chryssikos, T., et al. (2020). Open reduction and decompression of atlantoaxial subluxation with basilar impression using the cervical management base unit. World Neurosurgery, 138: 129-136.
