Focused ultrasound shows great promise for treatment of an aggressive brain tumor
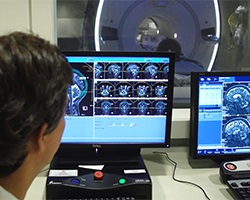
With the help of an innovative clinical trial, researchers and clinicians at the University of Maryland Greenebaum Comprehensive Cancer Center (UMGCCC) deliver outstanding outcomes for patients with brain cancer.
Rick Miller was diagnosed with a glioblastoma in 2019, an aggressive, rapidly fatal brain cancer. He was informed that the average survival time is a little more than a year. Miller started his care at UM Shore Regional Health, and through the UM Cancer Network, he was able to enroll in UMGCCC's phase one focused ultrasound clinical trial that changed the trajectory of his life and disease. The blood brain barrier therapy for brain tumor is not commercially available at this time, but the team is working to open more clinical trials in the near future. Today, his scans show no trace of cancer. That's true not only of 65-year-old Miller, but of many of the 14 other people who were enrolled in the initial clinical trial as well. Miller's case stands out because he is not only alive, but he is enjoying a great quality of life. He is back to work on a limited basis, and he has returned to the leisure and travel activities he loves.
Glioblastoma has historically been a nightmare diagnosis: a fast-growing cancer in the brain with a high rate of recurrence and a five-year survival rate of just five percent. Whereas existing therapeutics were of limited success, focused ultrasound (FUS) is a promising new therapy – among the first in recent years.
FUS is a noninvasive procedure that can circumvent the blood-brain barrier (BBB), which has historically limited the efficacy of chemotherapy for brain cancers. The blood-brain barrier protects the brain by preventing germs, toxins, and other harmful substances from entering. This protective capability, however, also blocks chemotherapeutic agents from reaching tumors in the brain.
University of Maryland Medical Center is a national leader in FUS. It is among the few institutions nationwide with deep expertise in the procedure, which it uses for movement disorders as well as brain tumors like glioblastoma.
FUS uses high-intensity ultrasound waves guided by high-definition MRI. During the procedure, the patient lies on a table in an MRI machine. There are two stages to the procedure: the planning (mapping) phase and the treatment phase. First, the treatment team uses the MRI to create a map of the brain to pinpoint the tumor's location and treatment targets.
Once the mapping process is complete, the treatment phase begins. Using MRI guidance, the team injects material into the patient's arm vein that forms tiny bubbles. The treatment team uses more than 1,000 high-intensity ultrasound waves, each individually tuned to travel through the skull to the tumor's precise location. The ultrasound waves activate the bubbles within the brain blood vessels, causing temporary opening of the BBB at the site of the tumor. This temporary opening permits the chemotherapy agents to reach the brain tumor cells. FUS does not use radiation and does not require any incisions.
"All we can say now is that we can do this safely," said Graeme F. Woodworth, MD, FACS, professor and chair of neurosurgery at the University of Maryland School of Medicine, who performed the FUS procedure on Miller. "Today, this is all quite promising. But we need more proof, there is more work to do."
Miller "stands out because he's done so well. He's not had any recurrence, but more importantly, he's maintained his quality of life and is getting back out and doing things he used to do before his cancer diagnosis," said Mark Mishra, MD, associate director of the UM Cancer Network and associate professor of radiation oncology at the University of Maryland School of Medicine.
The University of Maryland Medical Center is continuing its groundbreaking FUS research for different therapeutic agents and different brain tumor types. In the future, FUS could enable clinicians to detect brain tumor biomarkers, which is currently impossible because the blood-brain barrier prevents tumor cells from entering the bloodstream. The team hopes that, eventually, many more people can benefit from the life-changing outcomes similar to Rick Miller.
To learn more about this innovative treatment, please call 410-328-3514.
Related Content
- Blood Brain Barrier Disruption for Direct Delivery of Agents to Tumors
- MRI-Guided Focused Ultrasound for Essential Tremor at UMMC

