Novel Placental Imaging: An Improved Indicator of Potential Neonatal Complications
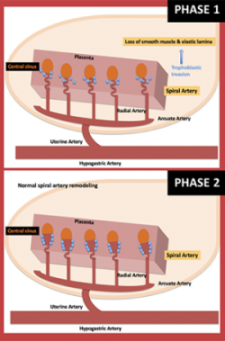
Normal uterine artery remodeling (UAR) is illustrated above. During pregnancy, trophoblasts invade the smooth muscle, vascular endothelial cells and elastic walls of the uterine spiral arteries to transform them into vessels that promote fetal blood flow. With abnormal spiral arteries, phase 2 is never fully attained, raising risk for later maternal-fetal complications.
An innovative way to image first-trimester placental blood flow could help determine which patients require early intervention to prevent later complications that could lead to neonatal morbidity or mortality. Perinatologists at the University of Maryland Center for Advanced Fetal Care (CAFC) pioneered B-flow/4D-STIC/M-mode, a multimodality imaging technique to gauge arterial distensibility – expansion of the vessel with increased blood pressure – and measure placental blood flow. 1
The imaging technique appears to be a promising addition towards the development of accurate, early detection of risk for preeclampsia, fetal growth restriction and preterm birth. Supported by a multi-year, $2.58 million NIH R01 grant, the team, led by Ozhan Mehmet Turan, MD, PhD, FACOG, professor of obstetrics, gynecology and reproductive sciences; director of fetal therapy and complicated obstetrical surgery; director of maternal fetal medicine; and vice chair for obstetric services at University of Maryland School of Medicine (UMSOM), tested the imaging in baboons to confirm it provides useful visual information and is now currently analyzing the data collected from using the technique for 300 patients. Their study is the first of its kind to explore placental function as a marker for neonatal risk.
Imaging the Placental Spiral Arteries to Diagnose Ischemic Placental Disease
Current models to predict which patients will face placenta-related pregnancy complications are lacking and still miss a large percentage of cases. It is now understood that abnormal uterine artery remodeling (UAR) is the earliest sign of ischemic placental disease (IPD), which alone accounts for about half of all preterm births. Given that IPD is also the pathology behind preeclampsia and fetal growth restriction, it would be of great benefit to detect it as early as possible, especially as the first trimester is of utmost importance to fetal development. The University of Maryland team believes this can be done through noninvasive imaging of the spiral arteries in the maternal placenta. In the presence of poorly adapted spiral arteries, blood is forced as a "turbulent jet" into the intervillous spaces, leading to inefficient and insufficient blood flow to the fetus.2 Suspecting that this condition could be measured by the inability of the maternal placental spiral arteries to widen in diameter during diastole, Turan sought a way to image this phenomenon.
"Doppler ultrasound shows us the limit of placental arteries, but it doesn't provide the information we need, which is knowing the distension of these arteries during the cardiac cycle," says Turan. "We wanted to see these arteries nicely dilating, then returning to their baseline," he continues. The distensibility of the spiral arteries permits the optimal hemodynamics that result in transfer of oxygen from the maternal to the fetal portions of the placenta. Turan was also interested in devising a way to measure the volume of blood passed through the spiral arteries, which have first-trimester diameters smaller than 1 mm, in case this might be later shown to have value for assessing placental health.
B-flow/4D-STIC/M-mode Visualizes Arterial Distensibility & Permits Blood Volume Measurement
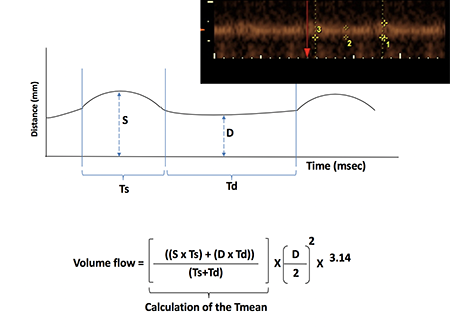
Through B-flow/4D-STIC/M-mode imaging (inset), blood flow and distensibility of placental spiral arteries can be viewed in real-time. From these images, the amount of blood passing through these arteries can be calculated.
To overcome the limitations of Doppler ultrasound in imaging spiral arteries, Turan developed B-flow/4D-STIC/M-mode in collaboration with other University of Maryland researchers. One of them was his wife, Shifa Turan, MD, associate professor of obstetrics, gynecology and reproductive sciences at UMSOM and director of the fetal heart program at UM CAFC, whose own research centers on imaging fetal hearts.
Through the use of varying grayscale intensities, B-flow ultrasonography allows for the visualization of blood flow through imaging dynamic changes in placental arterial size. Combined with 4D spatio-temporal imaging (STIC), a modality developed nearly two decades ago for fetal echocardiography that produces 3D renderings, and M-mode ultrasonography, which records rapid motion, B-flow shows real-time distensibility in spiral arteries. The series of modalities also provided the information required to calculate blood volume
Studying Baboon Placentas to Confirm Imaged Distensibility Correlates with Uterine Artery Remodeling
To find pathological evidence that would confirm that distensibility as viewed on ultrasound indicates uterine artery remodeling health, the team turned to a primate model. Elevated estradiol levels in baboons in early pregnancy results in abnormal UAR, so one group of primates was treated with estradiol and another group was not. As hypothesized, when the baboon placentas were imaged with the novel technique, the uterine spiral arteries showed more distensibility in the non-estradiol group. The placentas were then physically removed from the baboons and spiral arteries hand-counted to determine which ones were well-adopted. The results showed a significant correlation between arterial distensibility as measured through imaging and actual UAR.
SPARTAN Study: Applying the Novel Imaging Technique to Human Pregnancies
The University of Maryland team conducted a preliminary study, the "SPARTAN study," to evaluate the B-flow/4D-STIC/M-mode technique's advantages in imaging human placentas. The study demonstrated that, in normal pregnancies, spiral artery distensibility increases with gestational age.1 One of the other findings in normal pregnant women among a subset of 43 previously nulliparous participants is that spiral artery distensibility varies between racial groups, which the team expects to report on at a future conference.
"If we can better understand the physiology of poorly adapted uterine spiral arteries, then we can perhaps fix the problem," says Turan. He hopes that eventually this new imaging technique will be added to maternal blood markers to arrive at a more accurate model in predicting high-risk pregnancies and also, perhaps, help indicate which patients would benefit by starting low-dose aspirin early in their pregnancies. Maternal-fetal specialists may one day be able to offer better outcomes for both mothers and babies by incorporating B-flow/4D-STIC/M-mode imaging into their practices.
To inquire about Ozhan Turan's research into placental blood flow, send an email or call 410-328-7830.
Related Content
- Center for Advanced Fetal Care at the University of Maryland Medical Center
- Fetal Therapy at UMMC
- Complex Obstetric Surgery at UMMC
- Fetal Heart Program at UMMC
- Physician Brief: Twin-to-Twin Transfusion Syndrome (TTTS) with Proximate Cord Insertions (PxCIs)
1Shannon A, Harmon C, Turan O. A novel real-time ultrasound technique to assess human spiral artery transformation in vivo: SPARTAN study. Am J Obstet Gynecol. 2020;222(suppl 1):S284-S285. doi: 10/1016/j.ajog.2019.11.449
2Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiologic consequences of conversion of maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473-482. doi: 10.1016/j.placenta.2009.02.009
